PVC-free blood bags one step closer to reality
After several years of research and international collaboration between universities, hospitals and private producers, an ambitious project has now reached its final milestone: Prototype testing and preparation for full-scale production. Primo has played a vital part of this project, which will improve patient safety in the years to come.
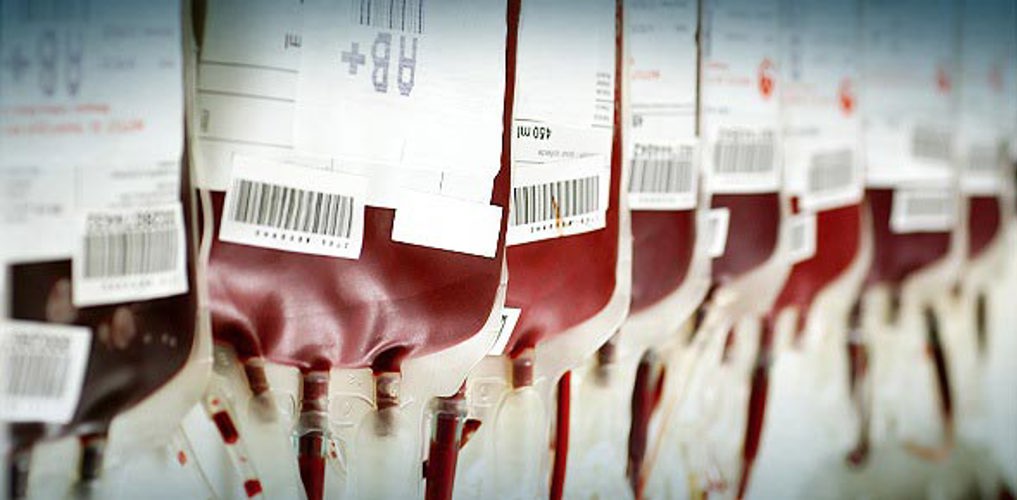
After several years of research and international collaboration between universities, hospitals and private producers, an ambitious project has now reached its final milestone: Prototype testing and preparation for fullscale production. Primo has played a vital part of this project, which will improve patient safety in the years to come.
The project was initiated in 2011, and since then the consortium has been working towards a series of milestones – not least of which was the testing and approval by external bodies of the prototype blood bags.
Currently, the status of the project is as follows:
- A completely PVC-free set of 4 bags has been successfully produced
- In-vitro tests of the bags ability to store red blood cells have been performed with satisfactory results
- The handling of the bags from donation to red blood cells have been simulated with water
- A Life Cycle Assessment has been carried out to demonstrate the difference between the new bag and today's bags impact on the environment
Ville Pitkänen, business area director for Primo Medico, says: “Our participation in this project is a source of great pride for Primo. When the final report on the project is released in 2017, we feel sure that the results will show that fullscale production is just around the corner – and we have been a vital part of this project and will have helped to increase patient safety in the future.”
Medical tubing by Primo
Blood bags are deceptively simple – just a bag, right? In fact, blood bags are highly complex products that consist not just of the bag itself, but of sampling devices, filters, joints, valves and tubes.
Primo’s contribution to the project has been to develop PVC-free medical tubing at our medical and clean room facility in Zory, Poland. Here, our extrusion and material experts have worked with scientists and doctors to develop the right compound and the proper production techniques to be able to deliver tubing for the prototype blood bags.
Why must it be PVC-free?
The global community uses vast quantities of blood bags every single day – in Sweden alone, almost half a million a year are used. And they are produced using PVC as a base material.
However well suited PVC may be for countless applications, it’s not terribly ideal for direct contact with delicate and critical biological matter such as blood plasma. The reason is that conventional blood bags - and the tubes needed - contain DEHP (diethylhexyl phthalate) as a plasticizer to make the end product soft; and DEHP is classified as a reproductive toxin. This is why DEHP is not allowed in products such as plastic toys, and the medical industry is searching for alternatives to remove softened PVC from direct medical contact.
The consortium behind the PVC-free blood bag project consists of both private companies and public entities such as the Karolinska University Hospital in Stockholm. The project has been realised with financial support from the EU’s Life+ programme.

About Primo
Headquartered in Copenhagen, the Danish group has sales and production activities at 11 locations in Europe and China. The group currently has 980 employees and a turnover of 205 million EUR (per 31.12.2022). The company was founded and owned by the Grunnet family and, since its beginning in 1959, has specialised in designing and producing customer-specific profile solutions in plastic.